Cold
Chain
Solutions
For
COVID-19
Vaccine
WHAT WE OFFER
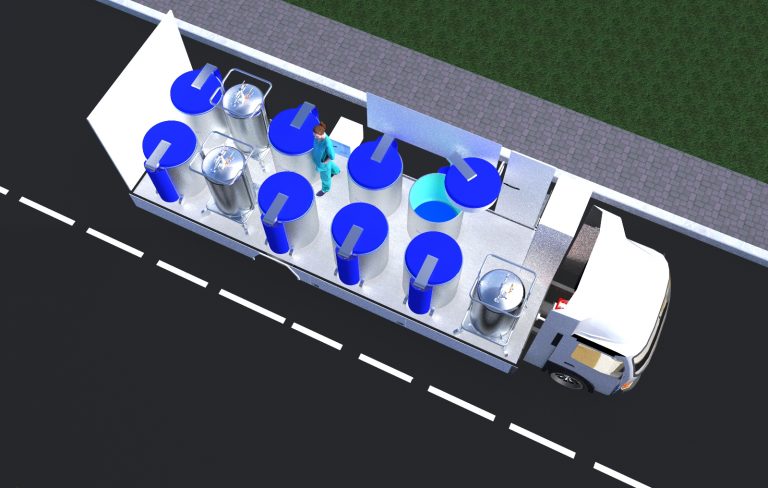
FULLY OPERATIONAL TRANSPORT SOLUTION
Our team at Biomedhelix has 15 years experience in the transport logistics of biological material at ultra low temperatures.
We have developed technologies and performed biological sample transports at storage temperatures of -80 °C in electrical freezers and temperatures below -135 °C in liquid nitrogen cooled storage vessels. These technologies were used in the following projects:
- Reallocation of Environmental Specimen Bank: more than 1 million samples were reallocated between two cities, 430 km apart.
- Several sample transports in electrical -80 °C freezers and in liquid nitrogen cooled storage vessels.
We are currently working on solutions for the transport and storage for the new COVID-19 vaccines that require a cold chain temperature below -75 °C that are suitable in the Africa context.
We are assessing liquid nitrogen sample storage technology and electrical freezer technology. We will develop a platform for the distribution and storage of COVID-19 vaccines at the required temperatures.



New Products for Ultra-low Temperature Vaccine Storage and Transport
BIOSAFE® DRYLOG LINES FOR THE SAFE HANDLING OF mRNA VACCINES
How it works:
Based on the liquid nitrogen storage system, we modified the interior to cool the system with dry ice. All advantages of the superinsulation will be available for the ultra-low temperatures (-70ºC) specified for the new mRNA vaccines.
We expect for the future, that we will be able to use liquid nitrogen for the cooling of the interior, having all the advantages then available for the vaccines:
- automatic refilling of the cooling medium
- lower temperatures will allow longer storage times for the vaccine
- independent from dry ice
In the meantime, we can guarantee the specified temperatures with the adapted interior. The vessel can be reconfigured easily for both cooling media.
Please find more information about the background of vaccine cold chain logistics in the video:
Technical Specifications
BIOSAFE® 420 MD (original lid)
BIOSAFE® DRYLOG CO2-Edition
Specifictions of original storage facilities for vaccines (according to mRNA manufacturers)
Qty of vaccine doses
96,000 (6 racks / 16 levels /1000 vaccine doses per level)
96,000 (6 racks / 16 levels / 1000 vaccine doses per level)
5,000 (5 trays à 1000 vaccine doses)
Holding time
30 d (4 weeks)
24 d (3.5 weeks)
10 d (1.5 week)
Consumption of dry ice (per 5,000 vaccine doses)
0.15 kg/d
0.18 kg/d
15 kg/d
Consumption of dry ice relative to mRNA manufacturers provided styrofoam insulated boxes
1:100
1:83
1:1
FIND MORE INFORMATION HERE
How We Operate
TRANSPORT MONITORING
- We implement multichannel temperature monitoring and GPS tracking.
Other systems on board:
- Oxygen level monitoring (for liquid nitrogen transport)
- Vibration monitoring (optional)


Storage Facilties
COVID-19 VACCINE STORAGE
We implement ultra low temperature storage solutions based on liquid nitrogen cooling
Other systems required:
- Oxygen level monitoring (for liquid nitrogen)
- Vibration monitoring (optional)
We implement ultra low temperature storage solutions based on electrical freezers






REFERENCES AND LITERATURE:
The team of Biomedhelix was involved in:
- https://www.biokryo.com/files/Download-Ordner/Englische%20Downloads/BioKryo-Cryotransports.pdf
- https://www.sciencedirect.com/science/article/pii/S1877282X09000411?via%3Dihub
- https://www.cryotherm.de/en/cryogenic-products-from-the-market-leader-at-a-glance-cryotherm/cryo-transport-transporting-goods-at-extremely-low-temperatures-cryotherm
